

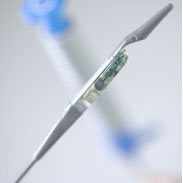
The Bravo™ reflux testing system measures actual pH levels in the esophagus, allowing you to provide an accurate, objective diagnosis.2
In the reflux care pathway, PPIs can disrupt the process of diagnosing GERD.1 They’re usually effective in managing acid levels, but the root cause of a patient’s symptoms may not be related to GERD.1 That’s the case for one in three patients on PPIs who still experience ongoing symptoms.1 Indeed long-term use of PPIs is now being associated with several adverse effects, such as kidney disease, bone fracture, osteoporosis, gastric polyps, pneumonia and dementia.3,4 The Bravo™ reflux testing system provides the accurate data you need to objectively diagnose — or rule out — GERD, allowing to optimize each patient's treatment.5
The Bravo™ reflux testing system has greater sensitivity than EGD and greater specificity than PPI trials.3 Its 96 hours of pH recording increases the identification of acid reflux episodes and the associated symptoms.6 And that's the information you need to understand if GERD is the underlying cause of your patient's symptoms.
Optimize or stop your patients' PPI therapy by confirming if they have GERD or not with Bravo reflux testing system.
Medtronic offers a synopsis of a clinical publication involving wireless pH monitoring. To get your copy of “Utilization of Wireless pH Monitoring Technologies: A Summary of the Proceedings from the Esophageal Diagnostic Working Group,” just fill out our quick form.

References
1. Herregods, T.V.K., et al. Patients with refractory reflux symptoms often do not have GERD: Neurogastroenterology & Motility. 2015;27(9): 1267-1273
2. Hirano I, Zhang Q, Pandolfino JE, Kahrilas PJ. Four Day Bravo pH Capsule Monitoring With and Without Proton Pump Inhibitor Therapy. Clin Gastroenterol & Hepatol 2005;3:1083-1088
3. Vakil N. Prescribing proton pump inhibitors: is it time to pause and rethink? Drugs 2012 Mar 5;72(4):437-45
4. Freedberg D, The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice from the American Gastroenterological Association. Gastroenterology 2017 152:706-715
5. Richter J, Pandolfino J, Vela M, et al. Utilization of wireless pH monitoring technologies: a summary of the proceedings from the Esophageal Diagnostic working Group. Dis Esophagus. 2013 Nov-Dec;26(8):755-65
6. Garrean CP et al. Acid Reflux Detection and Symptom-Reflux Association using 4-Day Wireless pH Recording Combining 48-Hour Periods Off and On PPI Therapy. Am J Gastroenterol 2008;103:1631-1637
US170989e
Bravo™ reflux testing system
Caution: Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner. Rx only.
Risk Information: The risks of the Bravo™ reflux testing system include premature detachment, discomfort, failure to detach, failure to attach, capsule aspiration, capsule retention, tears in the mucosa, bleeding, and perforation. Endoscopic placement may present additional risks. Medical, endoscopic, or surgical intervention may be necessary to address any of these complications, should they occur. Because the capsule contains a small magnet, patients should not have an MRI study within 30 days of undergoing the Bravo™ reflux test. Please refer to the product user manual or medtronic.com/gi for detailed information.